STEELHEAD TROUT AND INTERGRATED MULTITROPHIC AQUACULTURE (IMTA)
This study investigated methods to promote small scale finfish aquaculture operations in New England with a focus on promoting year-round revenue for current and future aquaculture operations. The first portion of the study was to investigate the use of a recirculating aquaculture system to raise steelhead trout at a water temperature and feeding rate that optimized growth rates and promoted healthy livestock. The use of the recirculating system was to identify if artificially maintained temperatures can increase biomass production in steelhead trout culturing compared trout pond culturing. The studied also investigate the use of an integrated multi-trophic aquaculture approach (IMTA), which permitted the culturing of different species at different trophic levels. The intention of investigating IMTA was to assess its ability to increase the revenue of small shellfish farms by diversifying the livestock species cultivated while mitigating any negative side effects associated with finfish culture (Troell et al., 2009; Wang et al., 2012). In New England, growers primarily grow and sell oysters. However, oysters in New England don’t grow for about four months a year once water temperatures drop below 10 degrees Celsius. Diversifying the species cultured by aquaculture operations in New England states could provide year round revenue and jobs.
Recirculating Aquaculture System (RAS) steelhead trout culturing experiment:
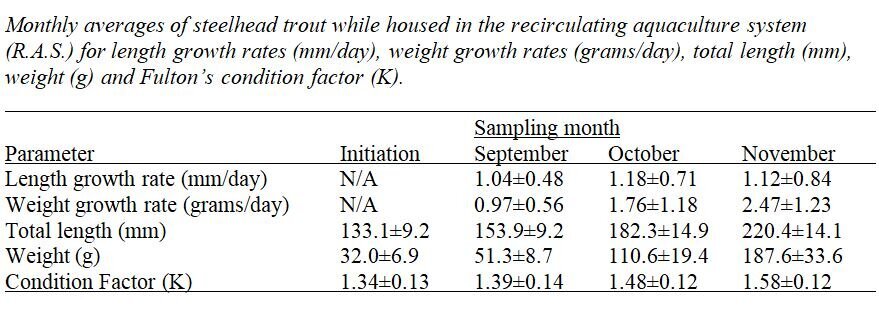
During the recirculating aquaculture system phase of the study, average monthly growth rates (grams/day) increased linearly (R-Squared = 0.9643) at a rate of 1.68% total body weight per day. The Fulton’s condition factor (K) of the trout in the recirculating system also increased linearly (R-Squared = 0.9999). Fulton’s condition factor is a widely accepted metric for estimating the health of fish, such as rainbow trout, and can be an indicator of stress, reproductive status, nutrition and age (Sharma and Bhat, 2015; Dekic et al., 2016; Shabani et al., 2018). The equation used to calculate Fulton’s condition factor (K) was: K = ((W)/(L*3))*100 (Froese, 2006; Tasaduq et al., 2011; Sharma and Bhat, 2015; Dekic et al., 2016). The use of Fulton’s condition factor (K) allows for health comparisons to other studies involving rainbow trout culturing and health.
During the three month period in the recirculating aquaculture system, total trout length increased at an average daily rate of 1.13 mm/day (SD±0.73). The monthly average growth rates (mm/day) of steelhead trout remain consistent throughout the recirculating aquaculture system phase, ranging from 1.04 mm/day (SD±0.48) in September to 1.18 mm/day (SD±0.71) in October.
The recirculating system was an efficient technique in promoting high growth rates and good health of cultured trout. During the three month recirculating aquaculture system experiment, the steelhead trout held within the system experienced linear growth in weight at a controlled temperature (20 Celsius) and a maintained feeding regiment (~2% total biomass/daily). Over the duration of the recirculating aquaculture system portion of the experiment, the average individual trout weight increased linearly (R - Squared = 0.9643), increasing by approximately 1.68% increase in body weight daily while housed in the recirculating aquaculture system.
Steelhead trout IMTA net pen experiment:
Two netpens were installed by Ward Aquafarms into two identical slips at Fiddler’s Cove Marina, North Falmouth, MA in December of 2019. Ward Aquafarms already utilizes two other slips for float storage from October-May. By placing the net pens adjacent to an existing shellfish farm operation, additional labor costs were be reduced, and integration into standard farm operations was easily established. The two identical surface net pens (4m x 6m x 3m; custom nets from Reidar’s Nets, New Bedford, MA), were hung from existing finger piers, utilizing existing flotation and cleats. Total weight and total length measurements were sampled monthly from January through April of 2020 to evaluate growth.
While housed in the net pen during from December, 2019 through April, 2020, Steelhead trout growth rates in length ranged from 0.50 mm/day (SD±0.47) in December, 2019 to 0.07 mm/day (SD±0.53) in January, 2020. Steelhead trout growth rates in weight (g/day) during the net pen phase ranged from 2.03 grams/day (SD±1.49) in January, 2020 to a daily loss in weight of - 0.32 grams/day in February, 2020. Additionally, The Fulton’s condition factor (K) ranged from 1.29 (SD±0.12) in December, 2019 to 1.64 (SD±0.16) in January, 2020 for steelhead trout housed in net pens.
By the end of the net pen portion of the study, the condition factor of the rainbow trout population transferred from the recirculating system had changed little, and even experienced decreases. On April 1st, 2020, after 118 days in the net pens, the condition factor of the trout population from the recirculating aquaculture system was 1.56 (SD±0.13), a slight decrease from their initial condition factor of 1.58 (SD±0.11). Given that the condition factor of the trout population did not increase during the net pen culturing, and actually decreased in some sampling months, any reductions in the condition factor of the trout while housed in net pens will be difficult to recover from. The higher the condition factor of a trout population upon deployment into net pens, the greater the buffer against any decreases in condition. Since the trout population experienced a continuous increase in its condition factor while housed in recirculating aquaculture system, increasing the amount of time the trout population stays in recirculating aquaculture system would make for a safer and more profitable net pen culturing.
While in the recirculating system the average trout weight nearly doubled every 30 days, increasing at an average rate of 1.88 grams/day (SD±1.48). Once the trout were transferred to net pens the average growth rate of 0.76 grams/day (SD±1.76). Reductions in weight gains of the steelhead trout while housed in the net pens was anticipated though, for reductions in water temperature are known to decrease growth rates in rainbow and steelhead trout (Myrick and Cech, 2000 and 2005; Richter and Kolmes, 2005). All significant gains in weight (i.e. biomass) were intended to occur during the recirculating system phase. Therefore, the only way to increase the size of the trout while being held in net pens pending sale during winter months is increase the size of the trout prior to transfer into net pens. Extending the duration in which the trout are housed in the recirculating aquaculture system would produce a larger, more valuable product for sales during the net pen holding phase.
IMTA kelp and shellfish:
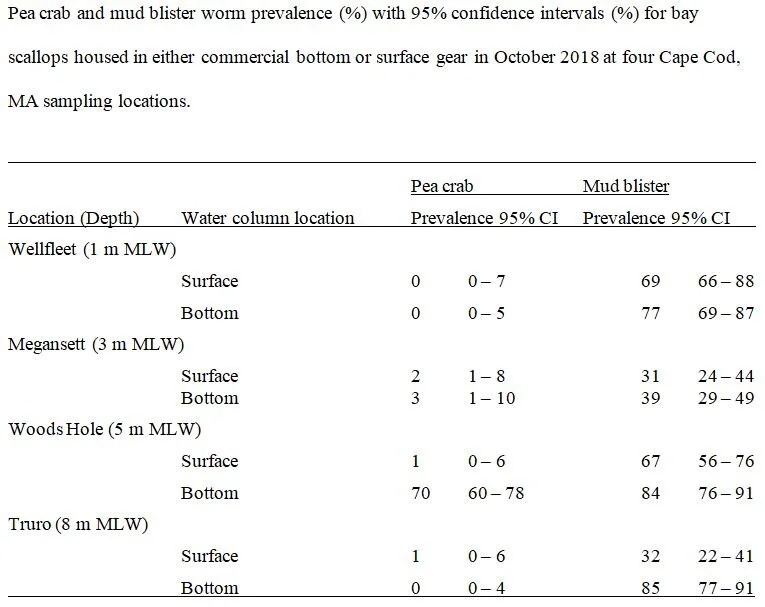
Oysters and bay scallops were stocked at two locations in December 2019: 1) directly adjacent to the trout netpens in the same slip; and 2) in outer Megansett at the farm growout site. Additionally, sugar kelp seed string was purchased from a locally available seed string supplier. Three, identical 20’ lines of sugar kelp were installed adjacent to both the trout net pens, and three identical lines were also installed at the current Megansett Harbor oyster farm. The lines were installed starting at the northwest corner, leading from north to south, spaced 25’ apart. The line was then sunk to 7’ at all locations.
The idea of practicing IMTA was mitigate any negative ecological impacts of the steelhead trout by using shellfish and macroalgae (sugar kelp) to consume excess nutrients while also producing extra aquaculture products (Troell et al., 2009; Wang et al., 2012). No significant differences were seen in growth rates and final shell heights of oysters and bay scallops housed near net pens in versus those housed at the control site.
A factor that likely influenced shellfish growth, or the lack thereof, was water temperature which remained below 7 degrees. Oyster metabolism and feeding rapidly decreases as waters temperatures drop below 10 degrees Celsius and bay scallop metabolism and feeding halts at temperatures below (Kirby-Smith and Barber, 1974; Barber and Blake, 1983; Dekshenieks et al., 1993; Comeau et al., 2008). Thus, the addition of oysters and bay scallops to Steelhead trout IMTA during winter months may add extra work with no economic gains.
However, the sugar kelp housed next to the trout net pens did exhibit significantly higher growth than the kelp at the control site.
With the ecological benefits associated with growing sugar kelp coupled with the significant growth, growing kelp next to steelhead trout net pens not only potentially mitigates negative side effects associated with finfish pen culturing but also provides an additional sources of revenue.
Sources:
Barber, B.J. and N.J. Blake. 1983. Growth and reproduction of the bay scallop, Argopecten irradians (Lamarck) at its southern distributional limit. Journal of Experimental Marine Biology and Ecology. 66(3): 247 – 256.
Comeau, L.A., Pernet, F., Tremblay, R., Bates, S.S., and A. Leblanc. 2008. Comparison of eastern oyster (Crassostrea virginica) and blue mussel (Mytilus edulis) filtration rates at low temperatures. Canadian Technical Report of Fisheries and Aquatic Sciences. 2810.
Dekic, R., Savic, N., Manojlovic, M., Golub, D., and J. Pavlicevic. 2016. Condition factor and organosomatic indices of rainbow trout (Onchorhunchus mykiss, Wal.) from different brood stock. Biotechnology in Animal Husbandry. 32(2): 229-237.
Dekshenieks, M.M., Hofmann, E.E., and E.N. Powell. 1993. Environmental Effects on the Growth and Development of Eastern Oyster, Crassostrea virginica (Gmelin, 1791), Larvae: A Modeling Study. Journal of Shellfish Research. 12(2): 241 – 254.
Kirby-Smith, W.W. and R.T. Barber. 1974. Suspension-feeding aquaculture systems: Effects of phytoplankton concentration and temperature on growth of the bay scallop. Aquaculture. 3(2): 135 – 145.
Myrick, C.A. and J.J. Cech Jr. 2000. Temperature influences on California rainbow trout physiological performance. Fish Physiology and Biochemistry. 22: 245-254.
Myrick, C.A. and J.J. Cech. Jr. 2005. Effects of temperature on growth, food consumption, and thermal tolerance of age-0 nimbus-strain steelhead. North American Journal of Aquaculture. 67: 324-330.
Richter, A. and S.A. Kolmes. 2005. Maximum temperature limits for Chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Reviews in Fisheries Science. 13: 23-49.
Shabani, F., Beli, E. and A. Rexhepi. 2018. Length-weight relationship and Fulton’s condition factor of rainbow trout (Oncorhynchus mykiss). Albanian Journal of Agricultural Science. 17(2): 261-264.
Sharma, R.K. and R.A. Bhat. 2015. Length-weight relationship, condition factor of rainbow trout (Oncorhynchus mykiss) from Kashmir waters. Annals of Biological Research. 6(8): 25-29.
Tasaduq, H.S., Balkhi, M.H., Najar, A.M., and O.A. Asimi. 2011. Morphometry, length-weight and condition factor of farmed female rainbow trout (Oncorhynchus mykiss Walbaum) in Kashmir. Indian Journal Fisheries. 58(3):51-56.
Troell, M., Joyce, A., Chopin, T., Neori, A., Buschmann, A.H., and J.G. Fang. 2009. Ecological engineering in aquaculture – potential for intergrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture. 297: 1-9.
Wang, X., Olsen, L.M., Reitan, K.L., and Y. Olsen. 2012. Discharge of nutrient wastes from salmon farms: environmental effects and potential for integrated multi-trophic aquaculture. Aquaculture Environment.